-40%
Portable Handheld Lensmeter Optometry Machine CE Certified Eye Care Professional
$ 147.3
- Description
- Size Guide
Description
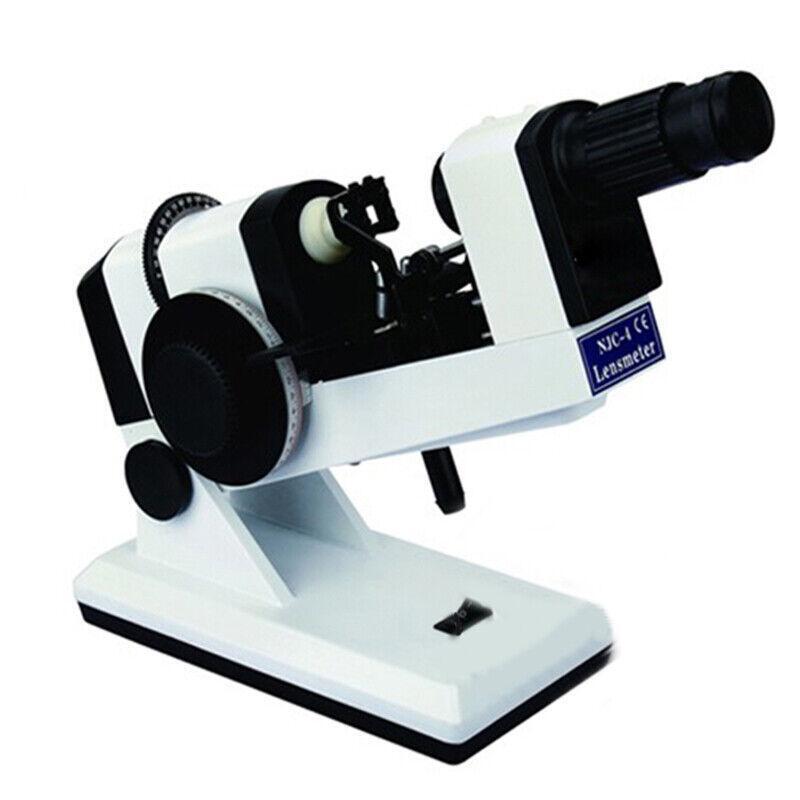
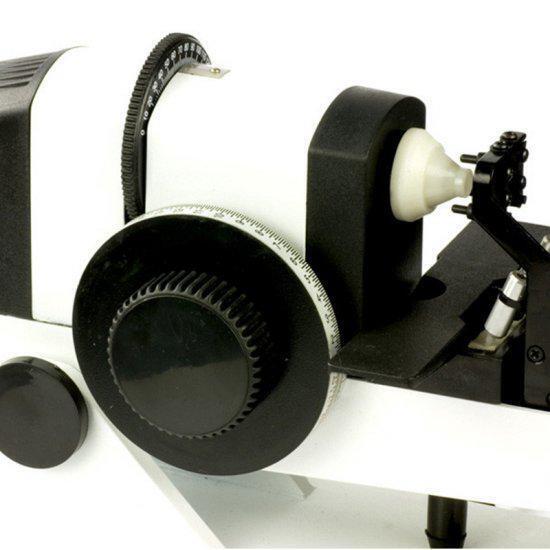
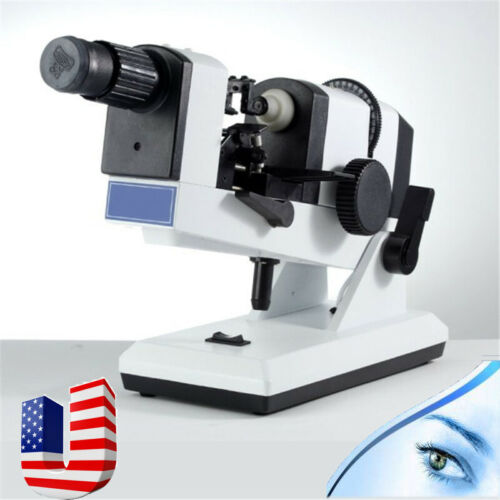
Description:This handheld lens tester is perfect for testing the lens, prism refraction, cylindrical axis, and more. It is AC/DC powered and offers precise readings with clear scales for easy use. The instrument is reliable, easy to operate, and comes with a compensating prism for accurate measurements. Ideal for glasses manufacturers, shops, and ophthalmology practices. Get your Portable Focimeter today!
Packing List:
1*Manual Lensmeter
Technical Parameters:
Eyepiece focusing range: ±5 D
The vertex refraction measurement range: -20D ˜ +20 D
The minimum measurement grid: 0.12D
The prism measuring range: 5Δ
The prism grid value: 1Δ
The prism axis range: 1--180º
Prism grid value: 1º
Measuring the maximum diameter of the lens: 20mm-85mm
The exchange: 220V / 110V - 6V, DC:3V
Net Weight:
5KG
FDA Disclaimer:
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
This item has been cleaned and treated according to the manufacturer's instructions.Eric-China-Beijing-86-15168779313
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
Ultrasound ,Ultrasonic Treatment Device is certified with the US FDA 510(k) Number:K161892
This item has been cleaned and treated according to the manufacturer's instructions.